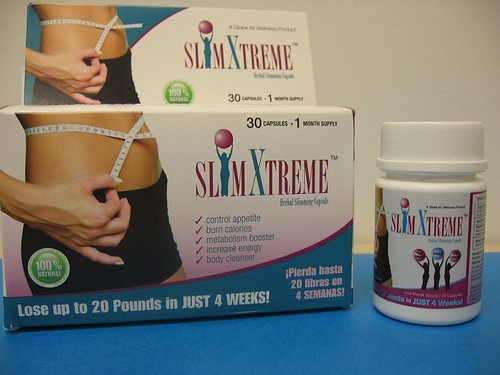
May 24, 2011 – Product Recall – Globe All Wellness, LLC Issues a Voluntary Recall of Dietary Supplement Found to Contain an Undeclared Drug Ingredient. For additional information, please refer to the company issued press release available on FDA’s web site at www.fda.gov/Safety/Recalls/ucm256649.htm
Posted by The U.S. Food and Drug Administration on 2011-05-25 15:00:58
Tagged: , Slim Xtreme Herbal Slimming Capsule , Undeclared Drug Ingredient , Globe All Wellness , LLC , Dietary Supplement.